Klotho: Alpha-Klotho and Beta-Klotho
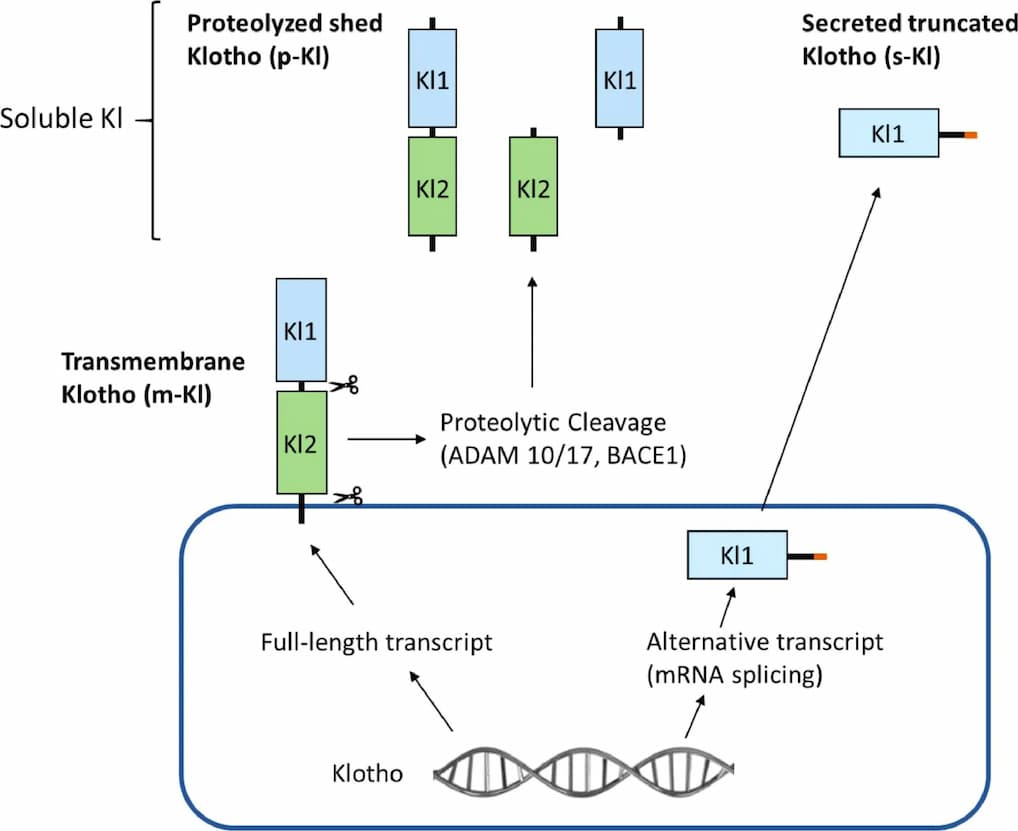
The klotho gene is composed of five exons and encodes a type 1 single-pass transmembrane glycoprotein (1014 and 1012 amino acids in mouse and human, respectively) that is located at the plasma membrane and Golgi apparatus. The intracellular domain is very short (~10 amino acids) without functional domains. The extracellular domain has two internal repeats, KL1 and KL2, which have amino-acid sequence homology to family 1 glycosidases that hydrolyze β-glycosidic linkage in saccharides, glycoproteins, and glycolipids. The linker region between two internal repeats contains four basic amino acids (Lys-Lys-Arg-Lys) that form a potential site for proteolytic cleavage.
Despite the sequence homology to glycosidase, glycosidase enzymatic activity is not detectable in recombinant Klotho protein probably because critical amino acid residues in putative active centers of the Klotho protein diverge from those of β-glycosidase enzymes. Indeed, Klotho exhibits weak β-glucuronidase activity in vitro and elicits biological effects through its β-glucuronidase and/or sialidase activity.
The extracellular domain of Klotho can be cleaved by membrane proteases such as ADAM10 and ADAM17 (ADAM metalloproteinase domain 10 and 17) and released into blood, urine, and cerebrospinal fluid. Cleaved Klotho functions as an endocrine, autocrine, and paracrine hormone on target cells. In addition, secreted Klotho is generated through alternative transcriptional termination of the klotho gene lacking exons 4 and 5 in mice. Secreted Klotho is detected in the blood, urine, and cerebrospinal fluid.
Klotho is expressed in multiple tissues and cell types and at particularly high levels in the kidney. Klotho is abundantly expressed in the distal convoluted tubule in the kidney and choroid plexus in the brain. It is also expressed in the renal proximal tubule], parathyroid gland and several sex organs including the ovary, testis and placenta. Recently, Klotho was found to be locally expressed in the adventitial area of the aorta, supporting the vascular protective effect of the Klotho protein. The list of tissue-specific expressions of Klotho is currently being updated.
Klotho has subsequently been characterized as one of a family of related proteins. These are all single-pass transmembrane proteins that include α-, β-, and ⋎- Klotho isoforms, the latter two discovered based on their homology with α-klotho.
α-Klotho comprises five exons and structurally its cognate protein is composed of a large extracellular domain followed by a transmembrane domain and a small domain of 11 residues comprising the intracellular C-terminus. The extracellular domain comprises two repeat sequences termed KL1 and Kl2 which are generated by full-length transcript splicing and can be cleaved by the metalloproteases ADAM-10 and ADAM-17. Cleavage of the extracellular domain results in a soluble form of Klotho being released. Soluble Klotho is the main functional form in the circulation and is detected in the blood, urine, and cerebrospinal fluid.
β-Klotho is mainly expressed in the liver, but is also found in the kidney, gut and spleen. It regulates the activity of members of the fibroblast growth factor (FGF) family, including FGF-21 and FGF-19.
⋎-Klotho is expressed in the skin and the kidney and has yet to be ascribed defined functions.